Research
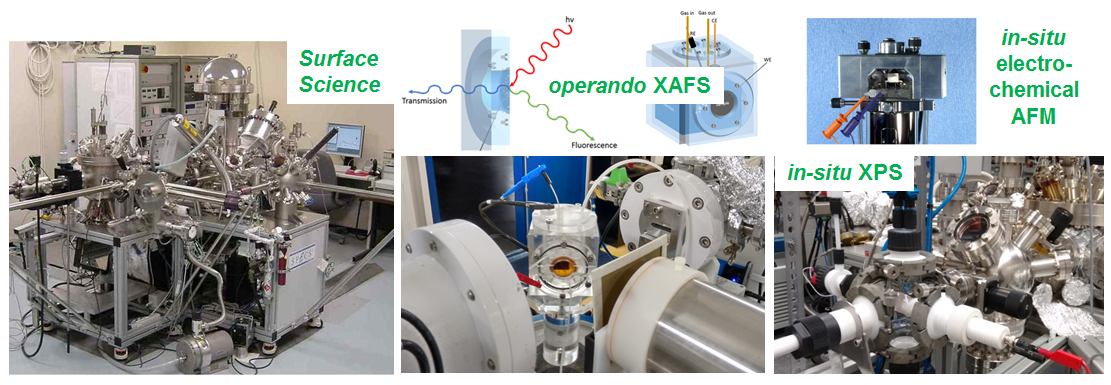
Our group investigates the structural, electronic, vibrational and chemical properties of size- and shape-selected nanostructures and their interfaces with gas and liquid environments. These properties are often significantly different from those of their bulk counterparts, strongly depending on morphological characteristics and environmental conditions. Understanding the interplay between the numerous factors which determine the physico-chemical behavior of such systems is crucial for optimizing the efficiency of their applications in fields such as energy conversion and catalysis. To this end, advanced synthesis methods and state-of-the-art, surface/bulk-sensitive characterization techniques, employed in situ and under operando conditions, are systematically used in our group.
The research within the Department of Interface Science focuses on:
The synthesis of nanostructures with well-defined morphological properties, tunable via controlled surface modification techniques (e.g. plasma treatment).
The study of chemical state, composition and structure-reactivity correlations in thermal catalysis processes under realistic operando reaction conditions. Here attention is being paid to systematically studying the effect of environmental conditions, surface adsorbates and support properties on the morphology, chemical state and electronic properties of nanoscale heterogeneous catalyst systems.
The investigation of the mechanisms underlying the electrocatalytic performance of nanostructures, in order to improve their reactivity and selectivity for desired products by tuning their structure, electronic properties and vibrational dynamics.
Nanostructure synthesis and surface modification
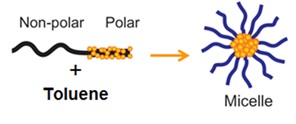
Size- and shape-selected metallic, monodisperse nanoparticles (NPs) are synthesized by using a colloidal synthesis method known as inverse micelle encapsulation technique. In this method, poly(styrene-b-2-vinyl pyridine) diblock copolymers consisting of a polar head (P2VP) and a non-polar tail (PS) are dissolved in a non-polar solvent (usually toluene) to form inverse micelles. |
| Read More |
Thermal catalysis
The performance of metallic nanoparticle catalysts for heterogeneously catalyzed reactions depends sensitively on the particle size, morphology and chemical state, as well as on the interparticle distance. These parameters can change significantly as a function of temperature, pressure and reactant flow during the catalytic reaction. Furthermore, interactions of the NPs with the support and the reactants (adsorbate effect) often result in a complex behavior which differs significantly from that of bulk materials or nanoparticles in vacuum. In the case of bimetallic systems, alloying and adsorbate-mediated segregation phenomena further complicate the situation, because they modify the elemental composition of the catalytically active surface. |
| Read More |
Electrocatalysis

In order to make clean energy storage and conversion technologies, such as fuel cells, water electrolysis cells, metal–air batteries, and CO2 to fuel conversion systems efficient nd economically viable, electrocatalysts with significantly enhanced activity and selectivity for desired products are required. Our group focuses on the development of high-performance, stable catalysts for electrochemical reactions of technological interest, such as the oxygen reduction reaction (ORR), hydrogen oxidation reaction (HOR), hydrogen evolution reaction (HER), oxygen evolution reaction (OER), and CO2 reduction reaction (CO2 RR). |
| Read More |